Simmons Citrate Agar- Composition, Principle, Uses, Preparation and Result Interpretation
 s Citrate Agar" width="650" height="360" />
s Citrate Agar" width="650" height="360" />
Simmons Citrate Agar is an agar medium used for the differentiation of Enterobacteriaceae based on the utilization of citrate as the sole source of carbon.
In the early 1920s, Koser developed a liquid medium formulation for the differentiation of fecal coliforms from the coliform group. Simmons later modified this formulation to produce a solid medium that eliminated potential errors when interpreting growth.
Ingredients per liter of deionized water:*
| Ingredients |
|---|
| Sodium Chloride (NaCl) | 5.0 gm |
| Sodium Citrate (dehydrate) | 2.0 gm |
| Ammonium Dihydrogen Phosphate | 1.0 gm |
| Dipotassium Phosphate | 1.0 gm |
| Magnesium Sulfate (heptahydrate) | 0.2 gm |
| Bromothymol Blue | 0.08 gm |
| Agar | 15.0 gm |
Deionized water = 1,000 ml
Final pH 6.9 +/- 0.2 at 25 degrees C.
Simmons Citrate agar is used to test an organism’s ability to utilize citrate as a source of energy. Ammonium Dihydrogen Phosphate is the sole source of nitrogen. Dipotassium Phosphate acts as a buffer. Sodium Chloride maintains the osmotic balance of the medium. Sodium Citrate is the sole source of carbon in this medium. Magnesium Sulfate is a cofactor for a variety of metabolic reactions. Bacteriological agar is the solidifying agent. Organisms capable of utilizing ammonium dihydrogen phosphate and citrate will grow unrestricted on this medium. If citrate can be used, the microbe will accumulate alkaline/basic byproducts.
Bacteria that can grow on this medium produce an enzyme, citrate-permease, capable of converting citrate to pyruvate. Pyruvate can then enter the organism’s metabolic cycle for the production of energy. Growth is indicative of utilization of citrate, an intermediate metabolite in the Krebs cycle.
When the bacteria metabolize citrate, the ammonium salts are broken down to ammonia, which increases alkalinity. The shift in pH turns the bromthymol blue indicator in the medium from green to blue above pH 7.6.
- It is used for the differentiation of Gram-negative bacteria on the basis of citrate utilization.
- Simmons Citrate Agar may be used to differentiate citrate-positive Salmonella enteritidis and members of Salmonella subgenus II, III and IV from the citrate-negative Salmonella typhi, Salmonella paratyphi A, Salmonella pullorum and Salmonella gallinarum.
- Simmons Citrate Agar is primarily used to aid in the identification of Enterobacteriaceae. Uses
include:
- Escherichia coli (usually -) and Shigella spp. (-) from other commonly encountered
Enterobacteriaceae (variable +).
- Edwardsiella spp. (-) from Salmonella spp. (usually +)
- Serratia proteamaculans (+) from Yersinia pseudotuberculosis (-) and Yersinia enterocolitica
(usually -)
- Klebsiella-Enterobacter groups (usually +) from E. coli (usually -)
- Proteus rettgeri (+) from Morganella morganii biogroups 1 and 2 (-)
- Yokenella regensburgei (+) from Hafnia alvei (-)
- Leminorella grimontii (+) from L. richardii (-)
- Acidovorax delafieldii (+) from A. facilis (-) and A. temperans (-)
- Dissolve above salts in deionized water.
- Adjust pH to 6.9.
- Add agar and Bromothymol blue.
- Gently heat, with mixing, to boiling until agar is dissolved.
- The medium may be used either as slopes in test tubes or as a plate medium in petri dishes. In both cases the surface of the medium is lightly inoculated by streaking and, where slopes are used, the butt of medium is inoculated by stabbing.
- For tubes, dispense 4.0 to 5.0 ml into 16-mm tubes.
- Autoclave at 121 degree C under 15 psi pressure for 15 minutes.
- Cool in slanted position (long slant, shallow butt).
- Tubes should be stored in a refrigerator to ensure a shelf life of 6 to 8 weeks.
- The uninoculated medium will be a deep forest green due to the pH of the sample and the bromothymol blue.
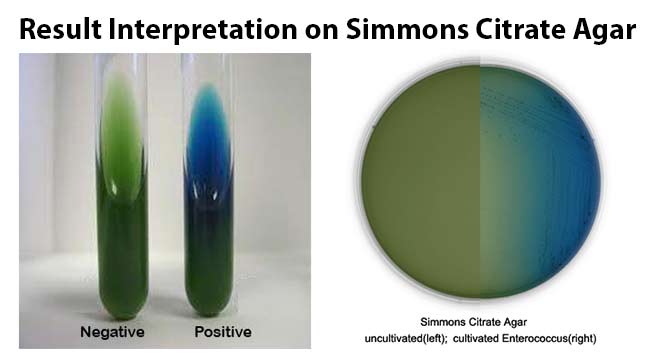
Result Interpretation on Simmons Citrate Agar
Positive growth (i.e. citrate utilisation) produces an alkaline reaction and changes the color of the medium from green to bright blue.
Examples: Serratia and the majority of the Enterobacter, Citrobacter, Klebsiella, Proteus and Providencia species, except Morganella morganii and Klebsiella rhinoscleromatis.
Negative test (i.e. no citrate utilisation) the color of the medium remains unchanged.
Examples: Escherichia coli, Shigella, Yersinia, Edwardsiella species, etc.
 s Citrate Agar" width="650" height="360" />
s Citrate Agar" width="650" height="360" />
- It is important not to carry over any nutrients into the citrate medium because this will result in false positive tests.
- Some organisms are capable of growth on citrate and do not produce a color change. Growth is considered a positive citrate utilization test, even in the absence of a color change.
- Tests with equivocal results should be repeated.
- The reactions of this medium alone are not sufficient for identification to the species level.
- The inoculum should be from a pure, overnight culture grown on a solid medium and not from a broth suspension.
Positive control: Klebsiella pneumoniae ATCC® 13883. Growth; color change of medium to blue
Negative control: Escherichia coli ATCC® 25922. Inhibited or no growth; no color change of medium
References
- Thermo Fisher Scientific Inc., Dehydrated Culture Media- SIMMONS CITRATE AGAR.
- Sisco Research Laboratories Pvt. Ltd.- Simmon’s Citrate Agar (59437).
- Austin Community College.
- Hardy Diagnostics- SIMMONS CITRATE AGAR (L80).
- Intuitive Systems, Inc.- Simmons’ Citrate Agar Slant.
- Scharlab- Simmons Citrate Agar (01-177).
- Merck Microbiology Manual 12th Edition- SIMMONS Citrate Agar.
- Dalynn Biologicals- SIMMONS CITRATE AGAR (TS62).
- Remel- Citrate Agar (Simmons).
- Conda Lab- SIMMONS CITRATE AGAR ISO 10273 (1014).
- Sigma-Aldrich, Inc.- SIMMONS CITRATE AGAR (S3681).
- HiMedia Laboratories Pvt. Ltd.- Simmons Citrate Agar (M099).
- Acumedia Manufacturers, Inc.- SIMMONS CITRATE AGAR (7156).
Similar Posts:
- Citrate Utilization Test- Principle, Media, Procedure and Result
- Salmonella Shigella (SS) Agar- Composition, Principle, Uses, Preparation and Result Interpretation
- Glycolysis Explained in 10 Easy Steps
- Malonate Test – Principle, Procedure, Uses and Interpretation

 s Citrate Agar" width="650" height="360" />
s Citrate Agar" width="650" height="360" /> s Citrate Agar" width="650" height="360" />
s Citrate Agar" width="650" height="360" /> s Citrate Agar" width="650" height="360" />
s Citrate Agar" width="650" height="360" />